IMI Audience Choice Award for COMBACTE-CARE Poster
During the Innovative Medicines Initiative (IMI)’s Symposium held on 22-23 October, Jan Feifel (Ulm University) received the Audience Choice Award for his poster ‘Time-to-event case-control designs: An efficacious tool for cohort studies on nosocomial infections when resources are limited’. Check out his poster below.
Jan Feifel is Research Assistant and Doctoral Student at the Institute of Statistics, Ulm University, Germany. In 2016 he joined COMBACTE CARE as a statistician and data analyst within the EUropean prospective cohort study on Enterobacteriaceae showing REsistance to CArbapenems (EURECA) prospective cohort study.
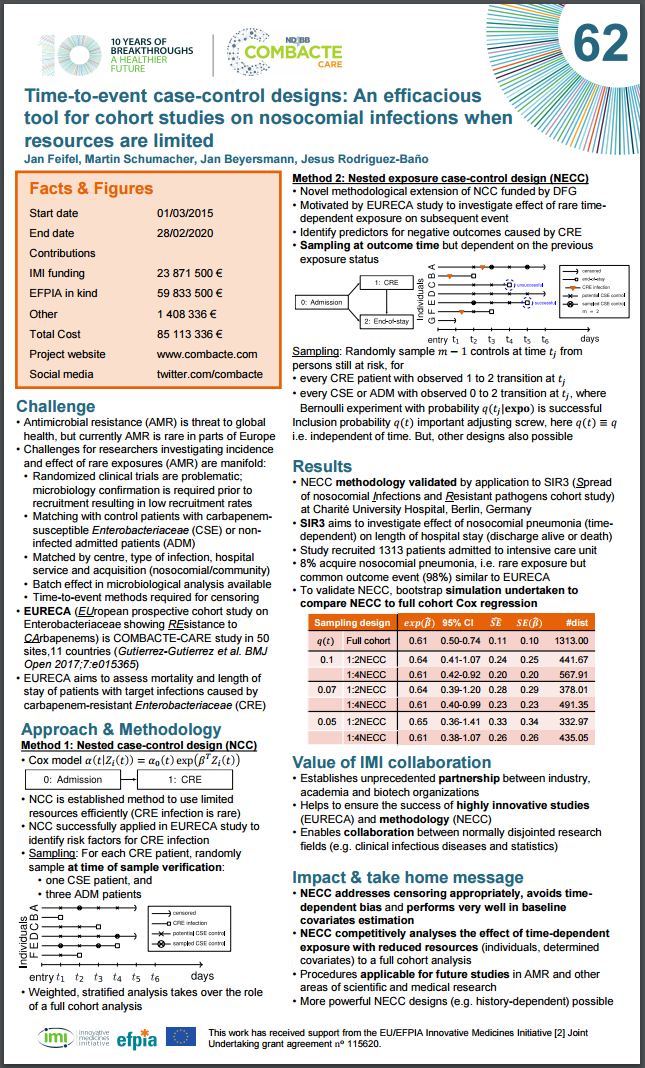
Jan Feifel on his poster: “To reach the objectives of EURECA innovative study designs have been chosen to deal with the rare incidence of infections due to carbapenem-resistance. Additionally, the underlying time-to event structure with time-dependent exposures proposes a strong challenge within the design and conduction stage.
For large cohort studies with rare outcomes (EURECA substudy 2) a nested case-control design has been chosen for an efficient use of limited resources. Here, small subsets of the individuals at risk that are randomly sampled dynamically over time. A stratified analysis takes over the role of the usual Cox full cohort approach.
Motivated by the evolved research question of EURECA substudy 3 we developed a non-standard nested case-control design, where the sampling probability evolves over time and may even depend on information already available to the researcher. Our design aims at a more precise estimation of the effect of exposure on mortality accounts for batch effects in a natural way and supports matching on additional variables. This is especially helpful as outcomes such as discharge are not necessarily rare, but interest lies in the rare impact of a time-dependent exposure such as the occurrence of an infection caused by carbapenem-resistance or disease progression. The nested exposure case-control design can be considered as a wise utilization of the available information in time and leads to a powerful and simultaneously less expensive procedure.
The audience choice was based on the poster itself and the presentation and explanation of the displayed content. In contrast to the presentation for the scientific committee, those explanations were more interactive for the audience.
All participants and all guest (public and invited) of the workshop were allowed to evaluate the contributions within a 30-minute time window. Each participant had two votes. One determining the best oral presentation and one for the best poster. For each category, the one contribution being selected the most was awarded.
The awards were presented to the awardees in a ceremony on Tuesday evening in presence of all participants. This was a dignified conclusion of the 10th IMI symposium.”
The 72 posters and 25 oral presentations were selected by a Programme Committee comprising top experts, and were clustered around four themes. The best presenters of the presentations and posters (as judged by the Programme Committee and the audience) received prizes at the end of the event.
The IMI 10th Anniversary Scientific Symposium marked 10 years – and more than 100 projects – that have been carried out to speed up the development of innovative medicines and transform medical research.
Last year Jan Feifel co-authored one of COMBACTE-CARE’s publications ‘EUropean prospective cohort study on Enterobacteriaceae showing REsistance to CArbapenems (EURECA): A protocol of a European multicentre observational study’. Read the publication here.
During the Symposium, other COMBACTE posters and presentations were held. An overview can be found here.
Related files
Related updates

EMA recommends Marketing Authorization for ATM-AVI
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion, recommending the gran...

Pfizer Receives Positive CHMP Opinion for its Novel Antibiotic Combination for the Treatment of Patients with Multidrug-Resistant Infections and Limited Treatment Options
COMBACTE is proud to announce a significant milestone achieved in COMBACTE-CARE. The European Medicines Agency (EMA) has recommended granting a market...