REVISIT
Determining efficacy and safety of ATM-AVI for treating serious infections caused by Gram-negative, carbapenem-resistant, bacteria. REVISIT – Revisiting serious bacterial infection with innovation is conducting a Phase III randomized, open-label, comparative clinical trial to determine the efficacy and safety of aztreonam-avibactam (ATM-AVI). ATM-AVI is intended for treating serious bacterial infections caused by Gram-negative bacteria, including metallo-beta-lactamase-producing MDR pathogens, for which there are limited or no treatment options.
The phase II REJUVENATE project (WP2A) reported the PK and safety of ATM-AVI in a representative patient population and confirmed the dose regime for Phase III program. WP2B is a pivotal regulatory submission global phase III study (REVISIT) which will further establish the efficacy and safety of treating a representative population of serious Gram-negative bacterial infections with ATM-AVI. Pfizer, as sponsor, and COMBACTE-CARE via WP2B are conducting this study in co-development with AbbVie (formerly Allergen) and co-funded by the US Biomedical Advanced Research and Development Authority (BARDA).
Current Status
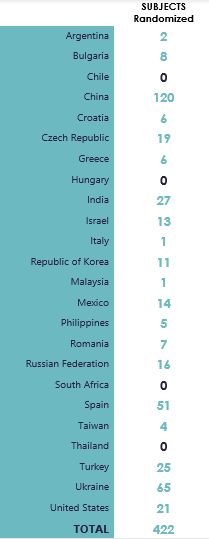
Globally 27 countries, (11 COMBACTE CARE countries) are participating in the REVISIT study. Currently site initiation is underway following completion of the first in a series of virtual investigator meetings held in June 2020. First subject is enrolled into the study, at site #1176 (Czech Republic), on 02 Sep 2020.
REVISIT Global Page
For an overview for the REVISIT trial global numbers, please visit this page.
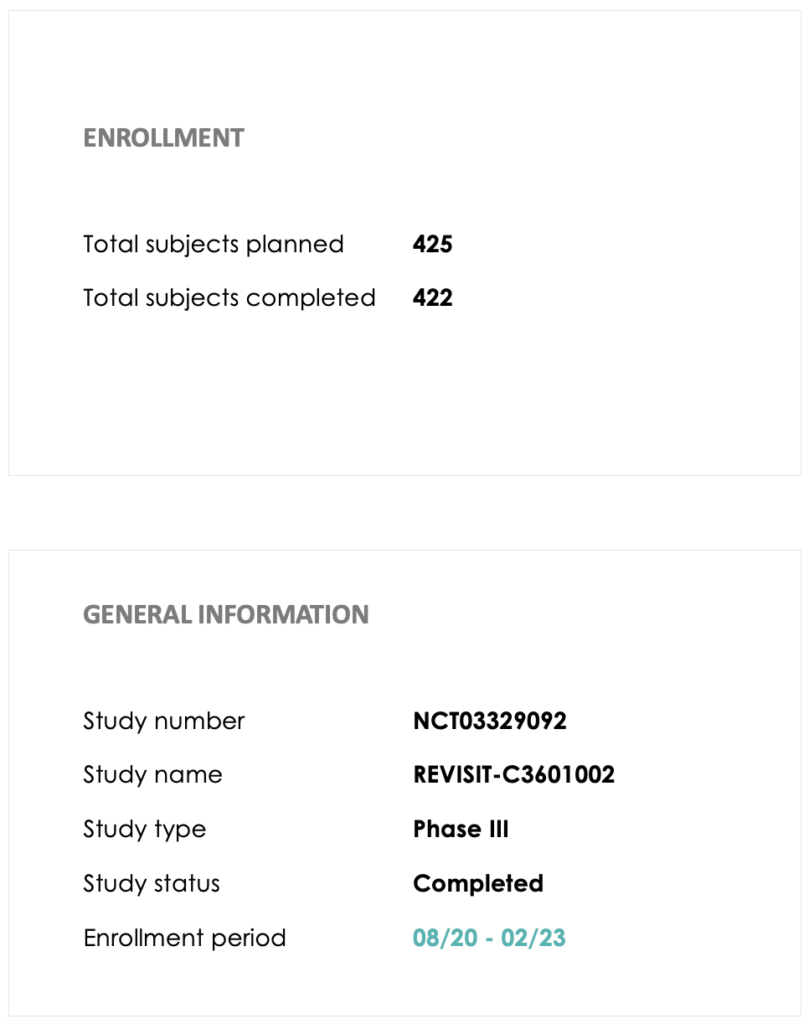
Study Level Information
Country Level Information
Study team members
-
Clara Rosso Fernandez
Sr. Project Manager / Academic / Hospital Universitario Virgen del Rocío
-
Clothilde Zimmerman
Project Manager / EFPIA / Pfizer
-
Francis Arhin
EFPIA / Pfizer
Study timeline
- Light blue
- Preparation phase
- Dark blue
- Trial period
Related updates

EMA recommends Marketing Authorization for ATM-AVI
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion, recommending the gran...

Pfizer Receives Positive CHMP Opinion for its Novel Antibiotic Combination for the Treatment of Patients with Multidrug-Resistant Infections and Limited Treatment Options
COMBACTE is proud to announce a significant milestone achieved in COMBACTE-CARE. The European Medicines Agency (EMA) has recommended granting a market...


